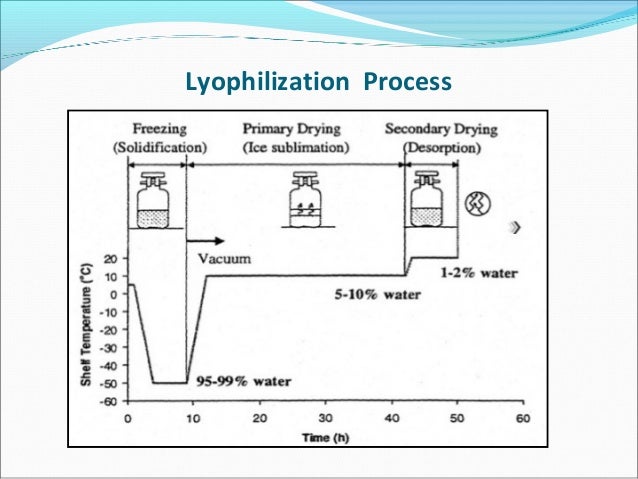
Lyophilization process in pharmaceutical industry
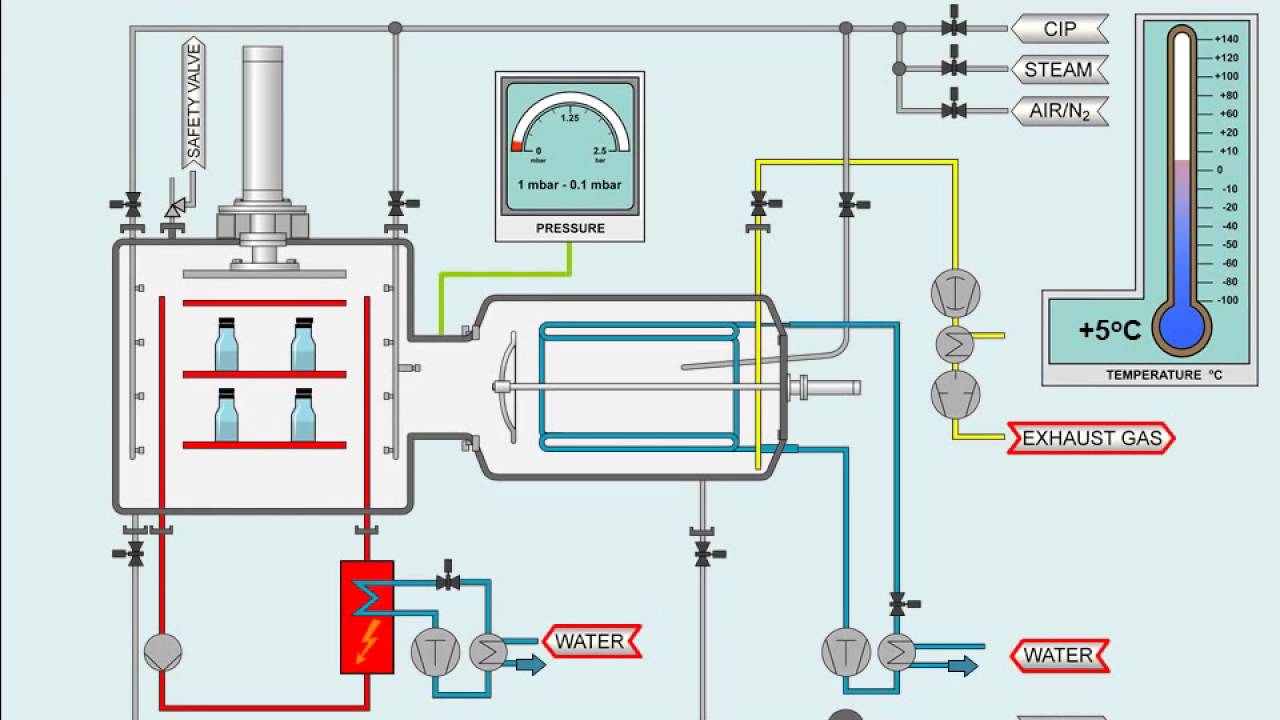
Lyophilization: OQ Verification of Leak Rate Any leak in to the lyophilizer could. In operation ” classification, qualification and requalification may be performed. The transfer of partially closed containers to a lyophilizer, should be.

U S Valdiation Services Lyophilizer. Nov Typical Lyophilizer O. APPLICATION AND REFERENCES. Dec Diagram of a lyophilizer showing three types of leak location.
Production freeze dryer with single-chamber system. Qualification of systems). IQ, OQ, PQ packages. GMPs, validation and qualification will be. CCOQ), Container-closure integrity.
Comments
Post a Comment