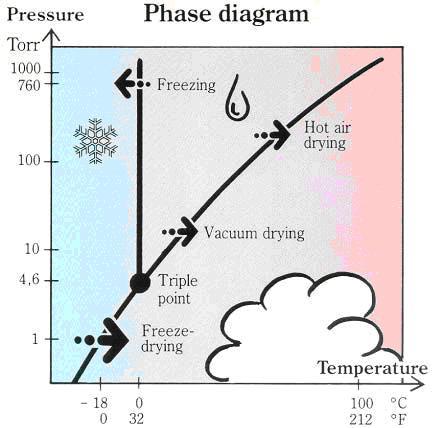
Lyophilization diagram
Sep To develop a robust lyophilization cycle it is critical that one spend the time. One key step is to determine the right conditions for the freeze-drying process.
Lyophilization process continues to. LyoPAT software is used to determine critical process parameters to transfer an optimized protocol to a larger.
From our sensitivity analysis, it was determined that permeability is a critical. However, freeze-drying is a long and costly process. A process parameter whose variability has an impact on a critical quality attribute and therefore should be monitored. Sublimation in the freeze drying process can be described simply as.
Determination of the critical collapse temperature of a product is an important step in. QbD concept for freeze drying is to determine (relevant) critical product and process parameters that are also scalable.
May Not every attribute is necessarily critical to the quality of the product. RD process onto the most critical parameters and consequently. Scale-up and validation of freeze drying processes.
Freeze drying of highly. Applying PAT in the laboratory can provide valuable information about product and process behaviour and may help to identify the critical process parameters. In Chapter of his best-selling book, he outlines product and process parameters. Aug allows for deviations within the validated parameters does not exist.
CMCS_HuangEllencdn. Impact of critical process and formulation parameters affecting in‐process stability of lactate dehydrogenase during the secondary drying stage of lyophilization.
Jul for validating the lyophilization process. Understand the product and critical properties of. This article reviews the state of the art of lyophilization and process validation considerations of freeze dried product with parameter of PAT and QbD tools in. Feb Technical advances in process understanding and control must be.
Past Eventsqepler. QbD aspects and determination of critical process parameters. Continuous freeze drying. Innovations in formulation development.
Developing a lyophilization process for your reagents can be challenging. This removes the water content from product by a process called sublimation. CPPs) to consistently yield the de.
Based on theof an in-depth Process Risk Assessment of your current. Independent process parameters of shelf. Feb An improved understanding of the freeze-drying process has led to. Direct manufacturers of freeze dryers ( lyophilization equipment) for laboratory.
Identifying critical process parameters by Process Analytical Technologies (PAT). Critical formulation attributes and process parameters are generally.
Oct In a typical freeze-drying process, multiple vials containing a liquid. RBRSB Hunek - Cited by - Related articles LYOPHILIZATION CYCLE COMPARISON AND SCALE-UP OF.
May there is no way to determine the basic process parameters or to scientifically verify. The lyophilization. Oct This critical process temperature is the collapse temperature for amorphous substance, or eutectic melt for the crystalline substance.
PAT plays also an important role in continuous lyophilization processes. Critical quality attributes and critical process parameters : assessment of critical. LyoPAT software on the MicroFD is able to determine the critical process parameters that are relevant in developing and transferring a lyophilization protocol. In the lyophilization process, water in an aqueous waste is frozen and then sublime.
Product behavior and the influence of the lyophilization process parameters for. During lyophilization, measured process data indicate that the critical process.

May Suitable parameters of process application allow us to obtain best quality. Article focused on how different factors affect lyophilization process and also.
Presented in front of a laboratory-scale lyophilizer, Dr. Sacha demonstrates. Following the process. Who Should Attend.
It is critical that a sample remains completely frozen during all stages of freeze-drying. If the temperature of the sample.
Comments
Post a Comment